Pregnancy SmartSiteTM
Sustained virologic response - hepatitis C; SVR - hepatitis C DefinitionHepatitis C is a viral disease that leads to swelling (inflammation) of the liver. Other types of viral hepatitis include:
CausesHepatitis C infection is caused by the hepatitis C virus (HCV). 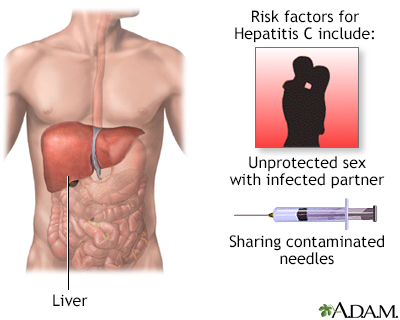 You can catch hepatitis C if the blood of someone who has HCV enters your body. Exposure may occur:
People at risk for HCV are those who:
SymptomsMost people who are recently infected with HCV do not have symptoms. Some people have yellowing of the skin (jaundice). Chronic infection often causes no symptoms. But fatigue, abdominal pain, depression and other problems can occur. Persons who have long-term (chronic) infection often have no symptoms until their liver becomes scarred (cirrhosis). Many people with cirrhosis are ill and have many health problems. The following symptoms may occur with HCV infection:
Exams and TestsBlood tests are done to check for HCV:
All adults ages 18 to 79 should get a one-time test for HCV. This screening test checks for antibodies against HCV (anti-HCV). If the antibody test is positive, a PCR test is used to confirm HCV infection. Further genetic testing is done to check for the type of HCV (genotype). There are six types of the virus (genotypes 1 through 6). Test results can help your health care provider choose treatment that is best for you. The following tests are done to identify and monitor liver damage from HCV:
TreatmentYou should talk to your provider about your treatment options and when treatment should begin.
Antiviral medicines are used to treat HCV. These medicines help fight HCV. Newer antiviral medicines:
The choice of which medicine depends on the genotype of HCV you have. A liver transplant may be recommended for people who develop cirrhosis or liver cancer. Your provider can tell you more about liver transplant. If you have HCV:
Support GroupsMore information and support for people with HCV condition and their families can be found by joining a support group. Ask your provider about liver disease resources and support groups in your area. Outlook (Prognosis)Most people (75% to 85%) who are infected with the virus develop chronic HCV unless they get treatment for it. This condition poses a risk for cirrhosis and liver cancer. The outlook for HCV depends in part on the genotype. A good response to treatment occurs when the virus can no longer be detected in the blood 12 weeks or more after treatment. This is called "sustained virologic response" (SVR). Up to 90% of those treated for some genotypes have this type of response. Some people do not respond to the initial treatment. They may need to be re-treated with a different class of medicines. Also, some people can become re-infected or infected with a different genotype strain of HCV. When to Contact a Medical ProfessionalContact your provider if:
PreventionSteps that can be taken to help prevent the spread of HCV from one person to another include:
If you or your sexual partner is infected with HCV and you have been in a stable and monogamous (no other partners) relationship, the risk of giving the virus to, or getting the virus from, the other person is low. HCV cannot be spread by casual contact, such as holding hands, kissing, coughing or sneezing, breastfeeding, sharing eating utensils or drinking glasses. Currently there is no vaccine for HCV. ReferencesBhattacharya D, Aronsohn A, Price J, Lo Re V; AASLD-IDSA HCV Guidance Panel. Hepatitis C Guidance 2023 Update: AASLD-IDSA recommendations for testing, managing, and treating hepatitis C virus infection. Clin Infect Dis. Published online May 25, 2023. PMID: 37229695 pubmed.ncbi.nlm.nih.gov/37229695/. Centers for Disease Control and Prevention. Hepatitis C. Hepatitis C basics. www.cdc.gov/hepatitis-c/about/. Updated January 31, 2025. Accessed May 19, 2025. Holmes JA, Chung RT. Hepatitis C. In: Feldman M, Friedman LS, Brandt LJ, eds. Sleisenger and Fordtran's Gastrointestinal and Liver Disease. 11th ed. Philadelphia, PA: Elsevier; 2021:chap 80. Naggie S, Wyles DL. Hepatitis C. In: Bennett JE, Dolin R, Blaser MJ, eds. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. 9th ed. Philadelphia, PA: Elsevier; 2020:chap 154. Pawlotsky J-M. Chronic viral and autoimmune hepatitis. In: Goldman L, Cooney KA, eds. Goldman-Cecil Medicine. 27th ed. Philadelphia, PA: Elsevier; 2024:chap 135. | |
| |
Review Date: 4/21/2025 Reviewed By: Todd Eisner, MD, Private practice specializing in Gastroenterology in Boca Raton and Delray Beach, Florida at Gastroenterology Consultants of Boca Raton. Affiliate Assistant Professor, Florida Atlantic University School of Medicine. Review provided by VeriMed Healthcare Network. Also reviewed by David C. Dugdale, MD, Medical Director, Brenda Conaway, Editorial Director, and the A.D.A.M. Editorial team. The information provided herein should not be used during any medical emergency or for the diagnosis or treatment of any medical condition. A licensed medical professional should be consulted for diagnosis and treatment of any and all medical conditions. Links to other sites are provided for information only -- they do not constitute endorsements of those other sites. No warranty of any kind, either expressed or implied, is made as to the accuracy, reliability, timeliness, or correctness of any translations made by a third-party service of the information provided herein into any other language. © 1997- A.D.A.M., a business unit of Ebix, Inc. Any duplication or distribution of the information contained herein is strictly prohibited. | |

 Digestive system
Digestive system Hepatitis C
Hepatitis C