Pregnancy SmartSiteTM
Cervix surgery; Cryosurgery - female; Cervical dysplasia - cryosurgery DefinitionCervix cryosurgery is a procedure to freeze and destroy abnormal tissue in the cervix. The cervix is the lower part of the uterus (womb) that opens at the top of the vagina. Abnormal changes in the cells on the surface of the cervix is called cervical dysplasia. DescriptionCryotherapy is done in the health care provider's office while you are awake. You may have slight cramping. You may have some amount of pain during the surgery. To perform the procedure:
An "ice ball" forms on the cervix, killing the abnormal cells. For the treatment to be most effective:
Why the Procedure Is PerformedThis procedure may be done to:
Your provider will help you to decide if cryosurgery is right for your condition. RisksRisks of any surgery are:
Cryosurgery may cause scarring of the cervix, but most of the time, it is very minor. More severe scarring may make it more difficult to get pregnant, or cause increased cramping with menstrual periods. Before the ProcedureYour provider may suggest you to take medicine such as ibuprofen 1 hour before the procedure. This may reduce pain during the procedure. After the ProcedureYou might feel lightheaded right after the procedure. If this happens, lie down flat on the examination table so that you do not faint. This feeling should go away in a few minutes. You can resume almost all of your normal activities right after surgery. For 2 to 3 weeks after the surgery, you will have a lot of watery discharge caused by the shedding (sloughing) of the dead cervical tissue. You may need to avoid sexual intercourse and using tampons for several weeks. Avoid douching. This can cause severe infections in the uterus and tubes. Outlook (Prognosis)Your provider should do a repeat Pap test or biopsy at a follow-up visit to make sure that all abnormal tissue was destroyed. You may need more frequent Pap smears for the first 2 years after cryosurgery for cervical dysplasia. ReferencesLewis MR, Pfenninger JL. Cryotherapy of the cervix. In: Fowler GC, ed. Pfenninger and Fowler's Procedures for Primary Care. 4th ed. Philadelphia, PA: Elsevier; 2020:chap 125. Nayar R, Chhieng DC, Crothers B, et al. Moving forward-the 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors and beyond: implications and suggestions for laboratories. J Am Soc Cytopathol. 2020;9(4):291-303. Erratum in: J Am Soc Cytopathol. 2020. PMID: 32565297 pubmed.ncbi.nlm.nih.gov/32565297/. Salcedo MP, Phoolcharoen N, Schmeler KM. Intraepithelial neoplasia of the lower genital tract (cervix, vagina, vulva): etiology, screening, diagnosis, management. In: Gershenson DM, Lentz GM, Valea FA, Lobo RA, eds. Comprehensive Gynecology. 8th ed. Philadelphia, PA: Elsevier; 2022: chap 29. | |
| |
Review Date: 3/31/2024 Reviewed By: LaQuita Martinez, MD, Department of Obstetrics and Gynecology, Emory Johns Creek Hospital, Alpharetta, GA. Also reviewed by David C. Dugdale, MD, Medical Director, Brenda Conaway, Editorial Director, and the A.D.A.M. Editorial team. The information provided herein should not be used during any medical emergency or for the diagnosis or treatment of any medical condition. A licensed medical professional should be consulted for diagnosis and treatment of any and all medical conditions. Links to other sites are provided for information only -- they do not constitute endorsements of those other sites. No warranty of any kind, either expressed or implied, is made as to the accuracy, reliability, timeliness, or correctness of any translations made by a third-party service of the information provided herein into any other language. © 1997- A.D.A.M., a business unit of Ebix, Inc. Any duplication or distribution of the information contained herein is strictly prohibited. | |

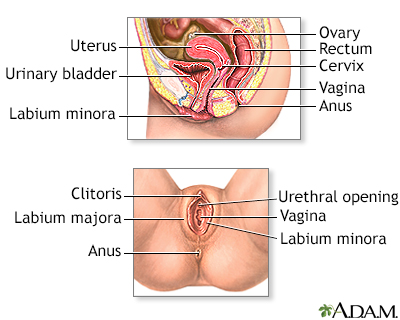 Female reproductiv...
Female reproductiv...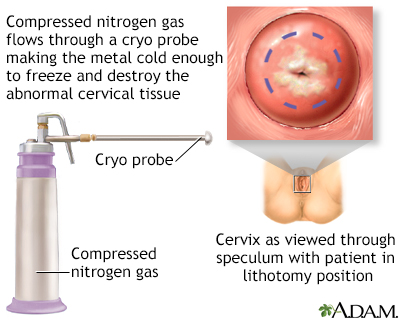 Cervical cryosurge...
Cervical cryosurge...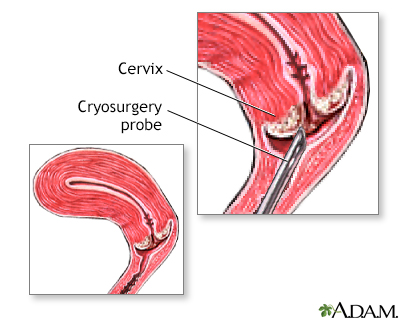 Cervical cryosurge...
Cervical cryosurge...