Pregnancy SmartSiteTM
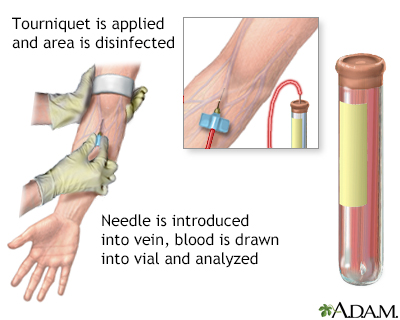
Bicarbonate test; HCO3-; Carbon dioxide test; TCO2; Total CO2; CO2 test - serum; Acidosis - CO2; Alkalosis - CO2 DefinitionCO2 is carbon dioxide. This article discusses the laboratory test to measure the amount of carbon dioxide in the liquid part of your blood, called the serum. In the body, most of the CO2 is in the form of a substance called bicarbonate (HCO3-). Therefore, the CO2 blood test is really a measure of your blood bicarbonate level. How the Test is PerformedA blood sample is needed. Most of the time, blood is drawn from a vein located on the inside of the elbow or the back of the hand. How to Prepare for the TestMany medicines can interfere with blood test results.
How the Test will FeelYou may feel slight pain or a sting when the needle is inserted. You may also feel some throbbing at the site after the blood is drawn. Why the Test is PerformedThe CO2 test is most often done as part of an electrolyte or basic metabolic panel. Changes in your CO2 level may suggest that you are losing or retaining acidic fluid. This may cause an imbalance in your body's electrolytes. CO2 levels in the blood are affected by kidney and lung function. The kidneys help maintain the normal bicarbonate levels. Normal ResultsThe normal range is 23 to 29 milliequivalents per liter (mEq/L) or 23 to 29 millimoles per liter (mmol/L). Normal value ranges may vary slightly among different laboratories. Talk to your provider about the meaning of your specific test results. The example above shows the common measurement range of results for these tests. Some laboratories use different measurements or may test different specimens. What Abnormal Results MeanAbnormal levels may be due to the following problems: Lower-than-normal levels:
Higher-than-normal levels:
Delirium may also alter bicarbonate levels. ReferencesBansal A. Respiratory acidosis, respiratory alkalosis, and mixed acid-base disorders. In: Johnson RJ, Floege J, Tonelli M, eds. Comprehensive Clinical Nephrology. 7th ed. Philadelphia, PA: Elsevier; 2024:chap 15. Seifter JL. Acid-base disorders. In: Goldman L, Cooney KA, eds. Goldman-Cecil Medicine. 27th ed. Philadelphia, PA: Elsevier; 2024:chap 104. | |
| |
Review Date: 5/19/2025 Reviewed By: Jacob Berman, MD, MPH, Clinical Assistant Professor of Medicine, Division of General Internal Medicine, University of Washington School of Medicine, Seattle, WA. Also reviewed by David C. Dugdale, MD, Medical Director, Brenda Conaway, Editorial Director, and the A.D.A.M. Editorial team. The information provided herein should not be used during any medical emergency or for the diagnosis or treatment of any medical condition. A licensed medical professional should be consulted for diagnosis and treatment of any and all medical conditions. Links to other sites are provided for information only -- they do not constitute endorsements of those other sites. No warranty of any kind, either expressed or implied, is made as to the accuracy, reliability, timeliness, or correctness of any translations made by a third-party service of the information provided herein into any other language. © 1997- A.D.A.M., a business unit of Ebix, Inc. Any duplication or distribution of the information contained herein is strictly prohibited. | |

 Blood test
Blood test